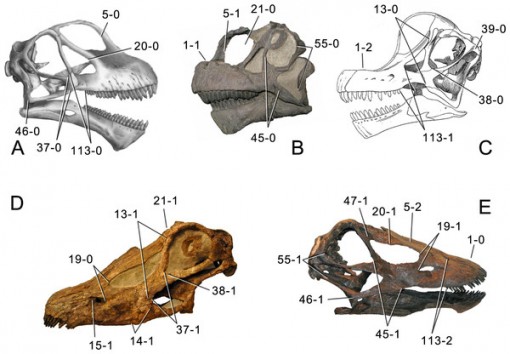
Skulls of different sauropods, showing the distinction between the long-snouted diplodocines,and the short-faced brachiosaurs and camarasaurs. (From Tschopp et al., 2015, Fig. 1)
The past few weeks have been abuzz with the media reaction to a just-published study by Emanuel Tschopp, Octavio Mateus, and Roger B.J. Benson analyzing the diplodocine sauropod dinosaurs and figuring out their classification and relationships. The study itself is a landmark in careful anatomical work, analyzing the problem specimen by specimen (a total of 81 specimens used) rather than generalizing based on previous clusterings of specimens, and looking at far more anatomical evidence than any previous study. Naturally, the press missed the significance of the study completely, and focused on just one minor point: they resurrect the genus “Brontosaurus” for specimens that had been lumped into Apatosaurus since 1903. ALL the publicity, and all the reactions of the non-paleontological reporters and readers was focused on this rather trivial issue, which is not important to real paleontologists in any way (except that we always get asked about it by the general public). Most of the reaction by sauropod paleontologists who were interviewed were generally favorable, but others were more cautious. Almost all agreed that is the most thorough work on the subject written to date, and it will be the foundation on which all future analyses will be built. Similar reactions could be found on the SVPOW (“Sauropod Vertebra Picture of the Week”) website, which is the main forum for discussion by specialists and amateurs about sauropods. (Even better: it was published in an open-access online journal, with access to the peer-reviewers’ comments as well!).
If Tschopp et al. are correct, the diplodocids were incredibly diverse during the Late Jurassic. In fact, in the Morrison Formation alone (Late Jurassic, mainly Colorado-Utah-Wyoming), they record FOURTEEN different species in nine genera (Suuwassea, Amphicoelias, Apatosaurus, Brontosaurus, Supersaurus, Diplodocus, Kaatedocus, Barosaurus, and Galeamopus) of diplodocines from a single formation that covers a limited geographic area and maybe 7-11 million years of time—plus several more specimens that have not been named yet. And that doesn’t count the non-diplodocid sauropods, including the monstrously huge Brachiosaurus, plus Camarasaurus, Haplocanthosaurus (the latter two are distantly related diplodocoids), and possibly several more. In a single quarry alone, Carnegie Quarry at Dinosaur National Monument (representing a single biological fauna and a short interval of time), they would recognize Apatosaurus louisae, Brontosaurus parvus, Diplodocus carnegii and D. hallorum, Barosaurus sp. among diplodocines, plus whatever taxon Carnegie Museum 3452 is (a sister-group to barosaurs), PLUS Camarasaurus and Haplocanthosaurus. That makes at least EIGHT distinct species of huge sauropods from a single interval of time, all crowding together and sharing common resources.
I realize that this is the cutting edge of sauropod research, and the thoroughness and rigor of the analysis are impressive. But as a paleontologist who has published taxonomy on large terrestrial vertebrates over 35 years, I found some issues troubling. This is an incredibly high diversity of animals for any limited time and place. If we were talking about species of small-bodied creatures, such as insects or rodents, there would be no problem. But huge land animals need LOTS of room to roam and feed. Ecological theory and empirical data show that larger species require bigger home ranges. The principle of competitive exclusion suggests that no two species can compete for the same resources, and that problem is magnified for larger animals, which rarely share territory with their own competing populations, let along closely related genera and species. We know of no examples of large land vertebrates today which exist in high diversity, and compete for the same resources. Even before poaching reduced their numbers, only one really big mammal (the elephant) lived in the savannas of Africa (the most diverse large land-mammal fauna today), and even they have problems if their populations exceed the carrying capacity of the habitat.
The same goes for the prehistoric past. Among communities with large terrestrial mammals, there are no instances of more than one or two huge species (usually an elephant or mammoth) in a fauna at the same time as another huge species, competing for the same resources. Even the vaunted “mammoth steppe” with its incredibly high diversity for an Ice Age tundra-grassland, still supported no more than one species of mammoth at given time and place. And sauropods were many times larger than elephants—yet a single time slice and locality (Carnegie Quarry) in the Morrison Formation (which had a similar semi-arid habitat to the African savanna, according recent analyses) allegedly supported eight different species of large sauropods.
What we are really talking about is the longstanding argument among taxonomists about “lumping” vs. “splitting”: “lumping” lots of different specimens in a single highly variable species vs. giving a different species name to every specimen which appears slightly different. The dispute goes back to the earliest days of taxonomy, when the pioneering collectors of the eighteenth and nineteenth centuries delighted in erecting new species willy-nilly, as sort of a mark of accomplishment, and often driven by the pride of adding to their collection. Such practice has been likened to stamp collecting: great fun, but not very scientific. All the early dinosaurs were named this way, which is why O.C. Marsh named one of his first diplodocines from Como Bluff, Wyoming, Apatosaurus (a juvenile specimen that was incomplete), and then a few laters named a more complete adult specimen from the same locality Brontosaurus. The latter specimen became the famous mount at Yale Peabody Museum, soon copied by the American Museum in New York and Carnegie Museum in Pittsburgh with their fossils, and all these mounts got the name “Brontosaurus” and made it a household name. But as early as 1903, paleontologist and lumper Elmer Riggs realized that these specimens were extremely similar, and that Apatosaurus was a juvenile “Brontosaurus”. By the rules of priority, Apatosaurus is valid and “Brontosaurus” became invalid. So it remained until just last month.
The splitters were dominant in the early twentieth century as well, but by the mid-twentieth century, the trend shifted. Such paleontologists as George Gaylord Simpson, who was also a pioneer in statistics in paleontology, argued that we should think of fossil samples like we think of modern populations of animals, and determine how many valid species there really are based on living biological analogues. Since then, most of the trend has been to lump the many invalid species created willy-nilly by pre-1940s paleontologists, and reduce the chaos of noise and bad taxonomy to get at a true biological signal of diversity. The famous and powerful paleontologist Henry Fairfield Osborn was a hyper-splitter who named nearly every specimen with the slightest differences from any other specimen a new species. His huge monographs on mammoths and elephants (1936) and the two-horned brontotheres (1929) are monuments of oversplit and just plain incompetent taxonomy, and both have been completely replaced by modern taxonomy which sank most of his invalid names. The hundreds of amazing skulls of the late Eocene brontotheres of the Big Badlands of South Dakota and adjacent states were once divided into many genera and dozens of species. Today, Matt Mihlbachler and colleagues recognize only one valid genus, Megacerops, with two species: M. kuwagatarhinus and M. coloradensis, and all the other famous names (still in the kiddie books) like Titanotherium, Brontotherium, Brontops, Allops, Menops, and others, are no longer taken seriously. When I started working on fossil rhinoceroses over 35 years ago, nearly every specimen with slightly different teeth was another species. But a large quarry sample of a single population from Trigonias Quarry in the upper Eocene beds of Colorado showed that all those variations in the crests and cusps of the upper premolars are due to intrapopulational variability, and since my 2005 rhino monograph all those long invalid names have vanished. Likewise, the gigantic indricothere rhinoceroses of Asia are now all in the genus Paraceratherium (replacing junior names like Baluchitherium and Indricotherium) for exactly the same reason: these huge animals would have been much like elephants with small populations roaming large distances to find enough treetops to browse on, and it’s extremely unlikely that more than one species could co-exist in the same place and time.
This is not to say that every recent study automatically leads to lumping. The hominid fossil record was once grossly oversplit, when only a handful of fossils were known and each one got its own name. Then during the 1960s, they went in for the trend toward lumping and perhaps overlumping on the grounds that only one human species can live on the planet today. But by the 1970s and 1980s, so many different fossils had appeared that there are more and more taxa which appear to be valid. Another example is a new paper I just published, where I recognize a whole new subfamily of extinct peccaries, the Hesperhyinae, with seven distinct genera (four new) and seven species (two new). But I based this analysis on a bunch of new unstudied specimens. In addition, I could show that there were male and females in each species (their tusks are distinctive), and I had large population samples of several species. I also accounted for the high degree of variability in the teeth, which are not as diagnostic as once thought (just as in the rhinos and so many other mammals). In addition, none of the species overlap in time or space, which is a strong grounds for distinguishing them—just as the co-occurrence in the same quarry is grounds for questioning whether more than one species is present.
Could this apparent high diversity of sauropods in the Morrison Formation also be due to oversplitting and failure to account for high intrapopulational variability? The biological and ecological arguments suggest this. To cite a similar example, Mihlbachler et al. (2004) did a careful study of African giraffes (long-necked, large-bodied animals a bit like sauropods), and found that they showed huge variability not only in cranial appendages (their horn-like ossicones), but in the proportions and shapes of the skull, features of the neck, etc.—precisely the same features that are used to justify so many different sauropod taxa. Yet giraffes are all one species (Giraffa camelopardis), with local geographic subspecies, but no one argues that they require different species or genera for these differences. Reading Tschopp et al. (2015) carefully, it appears that they have apparently accounted for juveniles and changes in shape due to growth of immature individuals, which is reassuring. But another possible source of variability might be sexual dimorphism, which I do not see mentioned at all in their paper. That is a tough issue, since it’s hard to tell whether fossils are truly male and female in most cases (except for those like deer where we have good modern analogues). A recent paper attempting to demonstrate sexual dimorphism in stegosaurs was harshly criticized on a number of grounds (not the least of which it used undescribed, unpublished specimens in private collections and not available for other scientists to study—a big no-no).
The dinosaur paleontologists are aware of the problem, of course, and there are a number of people who have speculated as to how explain so many closely related gigantic herbivores in the same place and time, when the resource base of the dry savannas of the Morrison Formation were a very meager food base to support even one gigantic herbivore. The most recent effort, by Button, Rayfield and Barrett (2014), argues that the skulls of these fossils are just different enough in their feeding mechanics that they could have specialized on eating different types of trees and foliage. Such studies assumes that the taxonomy is valid and there really were a large number of huge species living together, and therefore this has to be explained somehow. Maybe that’s plausible, but it still doesn’t fully address of how so many different species of gigantic animals, with monstrous appetites, made a living at the same time and place on just the foliage of tree tops and maybe some ferns. Remember: this is the Late Jurassic, when there were no flowering plants or fruits, no grasses, and most of the trees were conifers and cycads with tough fibrous slow-growing needles and fronds, not rapidly-growing nutritious leaves and fruits of flowering plants.
So before everyone begins the big party for “Brontosaurus” and celebrates this huge diversity of sauropod names, let’s hold our horses. Think outside the box for a moment. Is there any way to look at the sample of specimens from Carnegie Quarry as a single population of diplodocines (possibly some are males and some are females), or are the anatomical features completely incompatible with this idea? Keep the example of the extreme variability of giraffes in mind. Until someone has convincingly addressed the issue, I’m going to put “Brontosaurus” in quotes and not follow the latest media fad, nor will I overrule Riggs (1903) and put the name in my books as a valid genus.










Since “Apatosaurus” and “Brontosaurus” were assigned by the same person (Marsh,) do the rules of priority apply? Recall that Louis Leakey originally used the name “Zinjanthropus” for what is now commonly accepted as “Australopithecus;” why was the former not allowed to stand, according to the rules?
Yes, the rules of priority apply, no matter who does the naming. It was common for splitters like Marsh to name numerous new critters, and later someone (in this case, Riggs) lumps them all together. In the Leakey example, “Zinjanthropus” was named in 1959, but Dart named Australopithecus in 1924, so if you think they are the same genus, then the older name has precedence. If (as most anthropologists believe today) “Zinjanthropus” is the same is Paranthropus (named by Broom in 1938), “Zinj” is STILL a junior synonym. ONLY if you can argue (as Leakey tried) that “Zinj” is truly a distinct genus from Australopithecus or Paranthropus can you justify keeping the name as a valid nomen. But almost no one accepts Leakey’s arguments on that matter.
I don’t think who the person who assigns the names matters to much – as long as it’s agreed whether it is one species or two. My understanding is Apatosaurus was discovered in 1879, and ‘Brontosaurus’ in 1881 – hence the earlier name stands.
I would imagine this is the same reason why we have Basilosaurus for an early whale, where a name like ‘Basilocetus’ would be more accurate (correct me if I’m wrong Don)!
That’s correct–priority means the first valid name, no matter who named it, and no matter how appropriate it is. Thus, we cannot get rid of Basilosaurus, even though it was a whale, not a lizard; Hyracotherium is not a hyrax relative, and so on. That’s the rules, folks…
Please take a few minutes to alter your html for printing so it doesn’t change the nicely, well-laid out and readable page into a big block of unreadable text w/o figures. NO ONE wants a webpage reformatted for printing, ever (except you maybe). Thanks!
Get it back and clone it already. I want my brontosaurus burger.
Well, I don’t know how this is really a problem. All the specimens are from the same region, place and time, meaning that there would have been at least pone herd of over 81 individuals in the same region. Doesn’t the fact that all the Dinosaur NM specimens are together show that there were enough resources for that larger number?
Apparently you didn’t read the article carefully. The rules of ecology state that resources are limited, and large herbivores need huge home ranges and huge amounts of fodder to survive. Other than these Mesozoic sauropods, there are NO instances of multiple species of many large herbivores living together at the same time; 2-3 mammoths is the best we can do, and they are MUCH smaller than sauropods. These principles have guided our understanding of how to designate species in paleontology for a LONG time. In fossil mammals, these rules are the reason that we don’t recognize multiple species of giant rhinos or mammoths living in the same area at the same time–they are all just part of one highly variable population. THus, I’m questioning whether all the subtle variations in the neck vertebrae and small details of the skulls of diplodocine sauropods really justify designating over a dozen species in many genera, when it violates the rules of ecology–especially because we know the Morrison world was a dry savanna with sparse vegetation of tough, fibrous, low-nutrition, slow-growing conifers and cycads, with NO fruits or flowers or flowering plants or grasses to sustain them.
Well, we seem to be referring to different topics. Yes, there might not be enough resources for the different species, but I’m making a different point. Sure enough there are hundreds of skeletons of Morrison sauropods, and the thing is, there must have been enough resources for all of them to have survived. By your thinking, the resources were to limited to support each of these individuals being different species, but they very easily could have been. The DNM skeletons aren’t all from the same layer, this being recognized in the early 1910s. Thus your 81 species might have been spread out over 5 million years, definitely enough time for them. All in all, the gomphotheres, mammoths elephants and mastodons lived in the same region over 5 million years, maybe even 2 million.
Again, you missed the point. Tschopp et al. found that there were not hundreds of skeletons–there are at best only 81 partial skeletons of ALL diplodocine sauropods from ALL formations around the world. And they didn’t say there were 81 species–at most 14 species in 9 genera. IF they were all spaced out in different levels of the Morrison Formation, and only one or two overlapped in the same time and place, it might be plausible that they are different species. But when so many different genera and species are reported from a small time interval (Carnegie Quarry), it’s EXTREMELY unlikely that they represent as many genera and species as Tschopp et al. suggest. That’s just simple ecology and population biology, which should guide all our taxonomic judgements about how to distinguish species and genera. And YES, most of the sauropod skeletons in the Carnegie Museum collections from DNM DO come from a single layer, Carnegie Quarry, which is a SINGLE point-bar deposit that could not represent more than a decade or two of time at most.
This paper mentions two sites where Mammuthus columbi, Stegomastodon mirificus and Cuvieronius tropicus occur together:
http://www.tandfonline.com/doi/abs/10.1080/02724634.1999.10011169
You missed my point. I said one or two large mammals of elephant size, which accounts for your examples. But if current sauropod taxonomy is correct, there were at least SIX to EIGHT huge closely related sauropods, which are MANY TIMES larger than elephants or mammoths. THAT is stretching the bounds of ecology too much, in my opinion.
On large prehistoric mammal herbivores not living at the same areas, how about the American formations where both mammoths, mastodons and gomphotheres are known? Mammoths and mastodons are also frequently found in the same areas in the US, for example the La Brea Tar Pits.
Columbian and woolly mammoths also appear to have overlapped in range, and even interbed, according to recent work.
And in Eurasia, it appears mammoth species coocurred with their “ancestral” species, such as wolly mammoths with steppe mammoths, and steppe mammoths with ancestral mammoths…
What about the issue of metabolism? Wouldn’t a high number of species in a small range imply a low metabolism for sauropods? But P. Martin Sander has found strong evidence for a high metabolism among juveniles and in adults, if not high at least elevated well above modern lizards. http://www.ploscollections.org/article/browse/issue/info:doi/10.1371/issue.pcol.v02.i22
And John M. Grady has found evidence for mesothermy.https://student.societyforscience.org/article/hot-blooded-dinos-try-lukewarm; http://phenomena.nationalgeographic.com/2014/06/12/dinosaurs-tuna-great-whites-echidnas/
Indeed! That is another topic I could have raised, but chose not to for length reasons, since it opens up a whole new can of worms. Most paleontologists I know agree that sauropods could not have been full-fledged endotherms, because (just like elephants), their low surface area/volume ratio would have meant enormous problems dumping their excess body heat. Hence, the various ideas about mesothermy or “inertial homeothermy”–constant body temperature without endothermic heat production simply due to their large mass (especially in the warm tropical world of the Jurassic). IF you argue that sauropods were endotherms, not only do you run in to the problem I just mentioned, but it would also imply that they would have required MUCH more in the way of food resources, further suggesting that there are too many named species of sauropods.
Not “stupidity”, but “absurdity”.
Sorry if you don’t like it, but the rules of taxonomy are not subject to what is popular. They are there to minimize pointless scientific arguments, not to appease people who learned the wrong name as children and don’t want to change. And the Rule of Priority may not always keep the familiar, popular name intact, but it works to prevent the endless squabbling over whose name is right that existed before taxonomic codes were drafted.
“By the rules of priority, Apatosaurus is valid and “Brontosaurus” became invalid.” How stupidly pedantic. No one likes “Apatosaurus” (which is just a baby Brontosaurus anyway) except those who think it makes them sound smarter because they ‘in the know’. To state that a word that does NOT convey meaning and no uses is the “real” word while a popular word that vividly conveys its meaning is not is just .. well I have no word for that level of stupidity.
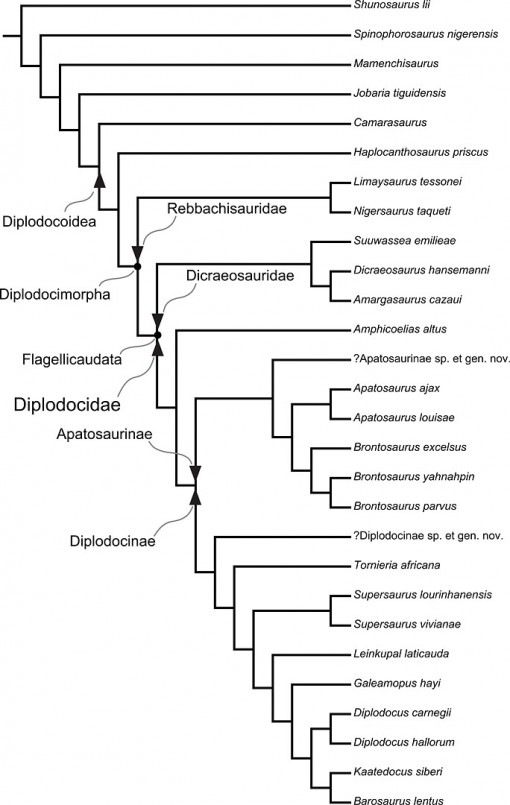
A slight quibble: Camarasaurus is not a diplodocoid, and has never been considered to be so. This figure is superficially deceptive – the authors do not label the node, they only label the diplodocoid stem (check the caption in Figure 120 in their paper, where this figure originally appears).
You could make a case for just two sauropod taxa (specifically including only one diplodocoid taxon) in the Morrison: a Camarasaurus-morph and an Apatosaurus/Atlantosaurus/Amphicoelias/Brontosaurus/Diplodocus-morph. In fact, this has already been done (http://www.dinosauriainternational.com/downloads/Brontodiplo_2011.pdf), albeit not in an official publication. However, as has been stated elsewhere (here: http://svpow.com/2010/10/07/the-elephant-in-the-living-room-amphicoelias-brontodiplodocus/ and here: http://svpow.com/2010/10/09/amphicoelias-brontodiplodocus-further-thoughts/), this route should only be taken with caution.
OK, I thought something was fishy when the camarasaurs were on that cladogram, since I’ve always understood them to be Macronaria, not Diplodocoidea. I assumed they were there as an out group, but the labeling is confusing.
And yes, I’m aware of that paper. Henry Galiano is a close personal friend of mine. I chose not to discuss it because of the widely acknowledged problems with the paper and its analysis (and use of specimens not in public access). But I think sauropod paleontologists should take a good hard close look at the idea of lumping most diplodocines, for the reasons I spelled out in the post, regardless of what you think of their analysis.
For a comparable but far less technical take on the subject, see this article by Paul Willis.
My own feeling is that it needn’t be controversial to treat ‘brontosaurus’ as an informal synonym of ‘apatosaurus’ if you’re not actually writing a scientific paper, in the same way that it’s perfectly acceptable to casually refer to your pet canis lupus familiaris as a dog.
And that’s how most paleontologists do it: “brontosaur” in quotes, but only Apatosaurus is underlined or italicized as a formal name.
Thank you, Donald. I knew you had been there many times – I just put up the link in case anyone else was interested.
As a fan of dinosaurs and paleontology since youth, I constantly wondered about some dinos being misinterpreted. I thought many dinos seemed very similar. I agree age differences and pride in willy nilly naming is most likely behind bronto. However, we do not refer to a caterpillar, as a butterfly until a certain age. Even though they are one and the same. Just being funny. Nice article.
To say that no one considers the different giraffes of Africa as distinct species is wrong. The leading work being currently done shows significant divergence in the 8-9 distinct types of giraffe.
http://www.biomedcentral.com/1741-7007/5/57
But this is based mostly on subtle molecular differences and their different spot patterns. Anatomically, the living giraffes are one highly variable morphology, with no big differences between subspecies in their bones or musculature. THAT is the kind of evidence that is relevant to my comparison to the oversplit sauropod dinosaurs. Molecular evidence is completely unavailable and irrelevant to the discussion here.
Molecular data is only irrelevant in that we don’t have any for sauropods. But the giraffe study shows that different species (or incipient species) may have only trivial osteological differences. Giraffes satisfy one of the two basic definitions of a “species” (animals that can’t interbreed/animals that don’t interbreed; the giraffe satisfies the latter). In captivity, different [sub]species can and do interbreed, but in the wild it’s extremely rare. Brown et al. (2007) postulate an interesting mechanism for the apparent speciation: natural selection for reproductive cycles that are synchronous with the dry season in different parts of Africa. The main variation is then seen in the pattern of spots. In any event, 5+ sympatric diplodocid species does seem a bit much.
Very interesting article, but one thing that troubled me as an ecologist. The Principle of Competitive Exclusion has only ever been experimentally demonstrated in microbial communities. It has never been demonstrated in vertebrate communities, but has been inferred from circumstantial evidence. Lizard communities in the deserts of Australia are incredibly diverse relative to desert lizard communities in South Africa and North America. Perhaps we just don’t know enough about the ecological conditions of Mesozoic North America to determine why or why not the sauropod communities could or could not be diverse.
” Even the vaunted “mammoth steppe” with its incredibly high diversity for an Ice Age tundra-grassland, still supported no more than one species of mammoth at given time and place. And sauropods were many times larger than elephants—yet a single time slice and locality (Carnegie Quarry) in the Morrison Formation (which had a similar semi-arid habitat to the African savanna, according recent analyses) allegedly supported eight different species of large sauropods.”
hm. How much time does Carnegie Quarry span?
btw, here is the svpow thread on it : http://svpow.com/2015/02/25/dinosaur-national-monument-quarry-map/
I can’t find a full pdf of the paper linked online, so if it says how much time the quarry spans, I don’t know.
I know the quarry well–seen it many times. The main bone bed is a single point-bar deposit, accumulated over a few years to a decade at most. For our purposes, that’s geologically instantaneous. Hence arguments about standing populations and ecological issues are relevant when they specimens are not time averaged or spanning an entire formation.
Are you sure all those species are from the Carnegie Quarry? Fossilworks lists the type locality of E. parvus as Carnegie Quarry E, Sheep Creek, Wyoming. This doesn’t seem to be the DNM quarry. I didn’t think D. carnegii was from the DNM quarry either (Fossilworks also lists that one as Sheep Creek Carnegie a quarry D). As far as I’m aware, the Carnegie Quarry at DNM contains only Barosaurus lentus, Apatosaurus louisae, and Diplodocus hallorum as far as diplodocids go. Of those, I can imagine Barosaurus being a dimorph of Diplodocus. I know Taylor et al. argued against this, but they cited differences between barosaurs from different horizons, which could be explained as evolutionary change in male Diplodocus rather than variation within a population of Barosaurus.
I think it’s the Morrison itself, not sauropods, that is too over lumped.
Also, isn’t the DNM Haplocanthosaurus a fragment of vertebra? Could it be a misidentified bit of something else?
Tschopp et al. (2015) list some CM specimens from Carnegie Quarry of D. carnegii.