Skeptic: Let’s start with the big questions. What is the problem to be solved? And why is systems biology the right method to find the answer?
Leroy Hood: The problem is this great complexity. Reductionism is the approach where you take an element of a complex system and study that element in enormous detail. However, studying one element in a complex system gives you no insight into how the complex system works. Systems biology highlights something extremely important—namely, biological networks underlie all of the complex responses and phenotypes of human beings. So, we first identify the network components and then study their dynamics. Systems biology takes a global, holistic view of a problem by thinking in terms of the networks that encode the information that is responsible for each phenotype, and so forth. The most fascinating part of the systems approach is that it can be applied to any kind of complex problem—physiological, psychological, or sociological.
Skeptic: Take DNA. Crick & Watson drilled down to the molecular structure—that’s reductionism. But then you have to build back out to the phenotype and the entire body, and how it interacts with systems both within the body and externally.
Hood: Correct. That’s systems biology. The first thing to figure out is what are the elements of information that DNA encodes—the genes. Once you’ve identified the 20,000 or so genes, you figure out how these genes connect to form these networks. Finally, you watch the networks operate during the dynamics of what you are studying. The really important thing about systems is that they operate across multiple scales. A system can be thought of at the level of one molecule, one cell, an organ, or at the level of the whole organism, and then you really begin to see how the various hierarchical levels operate differently in space and time.
Nathan Price: There has been tension between molecular biologists and systems biologists, especially in the early days, because molecular biology sometimes can feel very satisfying and concrete: “Here’s the protein…and here is its sequence.” In contrast, when building a system, you often see very complex relationships amongst all these.
Skeptic: OK. Let’s consider weight loss and diets. Why are diets faddish? And during a particular fad, why does a particular diet work for some, but not others?
Price: Let me give a specific example. First, studies in which we compared people who went on to lose weight with those who didn’t. In the thousands of measurements we’ve made, was there anything predictive about whether or not people would lose weight? When we looked at metabolites and proteins—once you normalize for BMI—nothing. But in the microbiome, two features were predictive.
The first was how fast your microbiome was growing. If your bacteria are growing fast, every calorie you eat is a calorie either for yourself or your microbiome. If your microbiome is consuming more, it is easier to lose weight. The second big factor is whether the genes in your microbiome are more likely to break down complex carbohydrates. Let’s say you eat a sweet potato. Some microbiomes will break that down into simple sugars that will spike your insulin more, making it harder to lose weight. But if you have a microbiome that will break down those same complex carbohydrates into short-chain fatty acids, it’s easier to lose weight. That was quite predictive and captured a fair amount of the variance between individuals.
Another big example is trying to lower something like LDL cholesterol. Some of that is genetically encoded and you can predict the blood level of LDL cholesterol from the genome, without knowing anything about a person’s diet or lifestyle. So we looked at whether people going through a wellness program could lower their LDL cholesterol without medication. If you had high LDL but your genome predicted low, you could lower it. But if your genome predicted high and you were high, you couldn’t. That is an incredibly useful tool that lets us know what you can change easily and what is going to be hard to change.
In short, we have a totally new method to look at your genetic potential versus your actual outcome. You get a roadmap of which lifestyle changes will make the greatest difference to your health. That’s big!
Skeptic: Hopefully, that kind of test will soon be available in every doctor’s office.
Hood: Exactly! That’s why I’ve proposed a second genome initiative where we take a million people for 10 years and conduct all these analyses. This will give us all the correlations for 150 different genetic risks, and all the correlations with the phenotype, so we can show unequivocally how this transforms the quality of your life. I guarantee that today we’re giving drugs to many people who should never be taking them. They could manage themselves just by diet and exercise.
Skeptic: You write that the 10 most popular drugs in the U.S. work for only about 10 percent of the people treated. Seriously?
Hood: Isn’t that absolutely striking? Yet it is true. A critical outcome of the million-person project is that we’ll have blood biomarkers that can tell us, unequivocally, which individuals are going to respond to particular drugs and which are not. That’s something pharma companies would hate because their bottom line likes this idea of one drug for everybody.
Price: Another factor is that your microbiome transforms about 13 percent of the drugs you take. That means you could be taking a drug, but if you have the wrong microbiome, it could change that compound so that you’re not even on the drug you think you are. This is a big problem that drug companies need to start thinking about systemically.
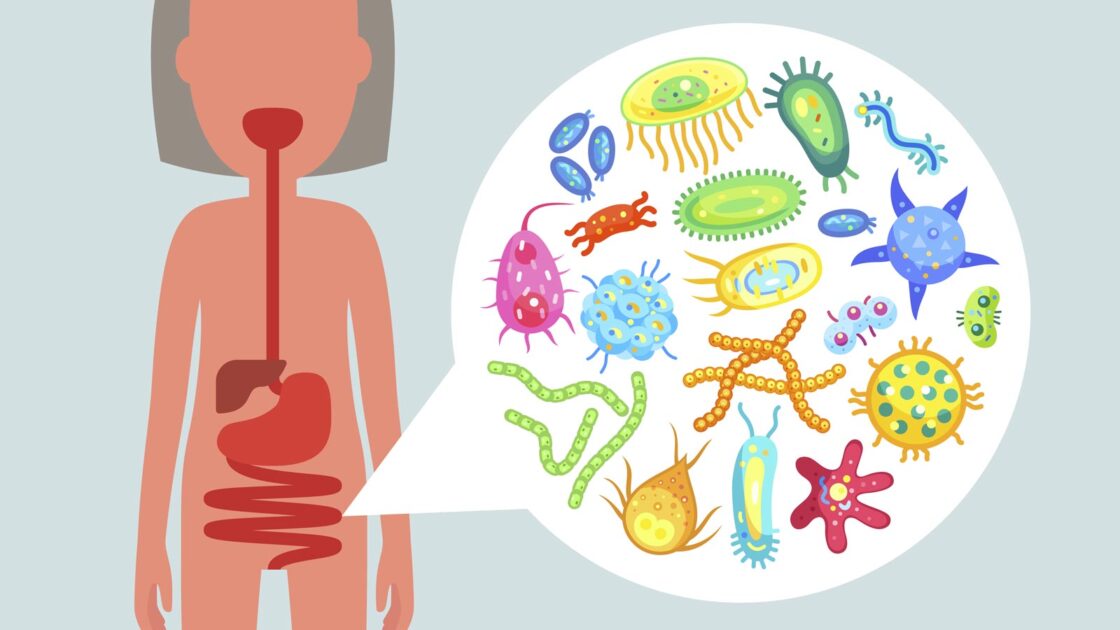
Skeptic: So, what exactly is the microbiome?
Price: Understanding the microbiome is one of the hottest areas in health and has just emerged quite recently. The microbiome is the bacteria and other small organisms that live on your skin and in your gut. Everything you take into your body—food, a supplement, a drug—passes through the microbiome before it gets to you. There are now tests that provide useful information about your microbiome.
For starters, you can see how your microbiome affects your digestion. You might have microbes that are making too much ammonia, which will cause your stomach to not be acidic enough to break down your food the way you need to. We can evaluate that. Recently, we ran a trial on people with Irritable Bowel Syndrome (IBS). They can now get a test, implement personalized interventions, and resolve their symptoms—in most cases—over the course of about a month.
The 10 most popular drugs in the U.S. work for only about 10 percent of the people treated.
Skeptic: Now that’s individualized medicine. When this knowledge and technology aren’t available, we have to resort to large-scale treatments. “Here’s the problem. Here’s our drug. Give it to everybody with that problem and hope maybe half get better.”
Hood: And today, thanks to the million-person genome project, the genome itself is going to reveal a whole series of diseases. For example, there are roughly 7,000 rare diseases. Many are single-gene defects. For each of those, we’re going to have to find a drug that works. And it’s going to be essential to examine the genome as early in life as possible because some cause disease during childhood or infancy. You want to know immediately which gene defects you have. Your physician will hopefully have these data and keep track of these things for you.
Skeptic: In that example, what would be the financial model for pharmaceutical companies?
Hood: First, the small molecule drugs today can only attack five percent of the proteins that exist in your genome. That’s a very small number. The pharmaceutical companies will need to generate lots of new drugs that can attack more than five percent. Second, they must figure out how to scale the research. If the clinical trial is going to cost three billion dollars per trial, it’s never going to work for individualized medicine. You need really efficient ways of generating lots of drugs and screening them effectively. Third, the federal government is probably going to have to help financially. A disease that’s devastating in infancy wipes out the productivity of that person for life. Avoid that and you have a productive, creative, functional citizen. You can make compelling arguments for being able to deal with these diseases at an appropriate time.
These are things pharmaceutical companies are just barely beginning to think about at a proper scale.
Skeptic: So we’re really at an intersection between academia, private industry, and government to make the transition from raw knowledge to application to industrial-size production of such kits and applications.
Price: We can progressively get access to information in ways that are simpler and cheaper than before. We already have the microbiome test and the blood measurement device that we hope will soon be approved in the United States. It’s already FDA-approved for a supervised blood draw, in which you need another person to stand next to you while you do it. We have successfully tested this in trials, including a big one of nearly 20,000 people with the University of Cambridge. So far, there have been no reported adverse events. And we have a 99.9 percent success rate of being able to get a measurement off the device when people use it at home. You can obtain your own blood sample and drop it in the mail.
Skeptic: This sounds somewhat similar to Elizabeth Holmes and Theranos.
Price: There are two different paths you can take on blood analysis. One is that you can try to get conventional clinical lab tests, which are typically done on large volumes of blood, and you try to miniaturize that. That’s what Theranos tried. And they failed. In fact, they were fraudulent all along the way, which is why Elizabeth Holmes is now in prison. Pretty much all other companies that tried to go down that route have also failed.
But there’s another path—the one that we’re pursuing—in which you use small volumes of blood to do what are called omics-based measures. These are your metabolomics (measuring all the metabolites out of the blood) or proteomics (measuring all the proteins). Those technologies, based on mass spectrometry or on capture agents, are only done on very small volumes.
We can make thousands of measurements out of that small volume. The challenge is how to interpret all of that information. Working with that data, tying it to health outcomes, connecting it to electronic health records, and monitoring people’s health is a much bigger challenge. It’s much more of an AI-data problem than anything else. And I’d much rather try to solve the information technology challenge than go down the Theranos road.
Hood: What Elizabeth Holmes projected is going to be done. I just want to say that it is a valid way of thinking about the technology. Technically, it’s far more difficult. But we are going to learn how. It’s going to take 10 or 15 years. It’s just a matter of getting appropriate, miniaturized measurements in microfluidics or nanotechnology.
Skeptic: Let’s talk about the future of medicine. What is CRISPR?
Price: CRISPR allows you to go into a genome and essentially edit any base pairs that you want. Incredibly powerful, it holds the potential to end all genetic-related disease, or at least monogenetic defects. Huntington’s disease is a compelling example. Unlike most genetic traits, it has essentially 100 percent penetrance. If you have the gene for Huntington’s, you will get Huntington’s disease. But you can CRISPR it out. Even in the embryonic stage! That eliminates the gene, and all your progeny forever will not carry it. That’s amazing.
Skeptic: What about stem cells?
Price: Stem cells are the body’s raw materials—cells from which all other cells with specialized functions derive. They are very powerful because they are generative. They grow all other cell types. Of particular interest are induced pluripotent stem cells which are programmed to grow tissue. And so, you put them in—in a way taking a leap of faith—trusting in the intelligence of the stem cells to act on their programs and to rebuild the right tissue. It’s all evidence-based, but we don’t fully understand the logic of how they do it.
Skeptic: What about cancer?
Price: Most of us may have already had cancers in our lives, but they started and then our immune system cleared them out. However, when you develop a tumor, your immune system didn’t recognize. The cancer fooled your immune system. Immunotherapies—not trying to kill the cancer off with a drug, but rather teaching the immune system to get the cancer that it missed—are one of the most exciting developments.
If you know the molecular properties of the cells that have become cancers and were missed by your immune system, you can create a vector that will look for certain gene expressions or molecular properties or some combinatoric aspect of those cells, then go in and initiate a program that will stick a molecule up from the outside of the cell. That molecule will act as a signal to macrophages, which are cells in the immune system that come and eat other cells, in this case destroying the tumor cells.
We have a totally new method to look at your genetic potential versus your actual outcome. You get a roadmap of which lifestyle changes will make the greatest difference to your health.
Skeptic: What about stories of injecting tumors with vaccines? Jimmy Carter was treated this way for his brain tumor.
Price: It’s a similar idea. You take pieces of the cancer cells, and then you create antibodies. You’re placing a signal into the immune system that says these are fragments of something foreign. Your immune system can then look for those specific tumor cells and kill them. It doesn’t yet work for the majority of cancers, but for the fraction that it does, it’s amazing.
Skeptic: Is part of the problem that there are so many different kinds of cancers, and they’re different in each body?
Price: Exactly. And that is why precision medicine is probably more advanced in cancer research than any other area. You can pull out the tumor and you can sequence it. You can look at its gene expression, its metabolites, and its proteins. You can take your genome and the genome of your cancer and design a map of exactly what’s different about those cells. You can do this on a per-person basis, and it’s at the core of individualized therapy.
I think the term cancer is a total misnomer. We should move away from terms like prostate cancer, lung cancer, or breast cancer. It should always be cancers. Cancers are a huge, massively heterogeneous, highly diverse set of diseases and conditions and molecular mutations, not at all a single phenomenon.
Skeptic: Starting today, what can each one of us do to be healthier?
Price: Exercise. You lose between zero and one percent of your muscle mass per year as you age. Decade after decade, that adds up. You become frail in your later years. There are things you really want to be able to do when you’re older—for example, to stand up from the ground without any assistance. When you’re young, that’s easy; when you’re older, it gets harder. If you lose that ability, you’re putting yourself at much greater risk. Balance and posture are also important. So are stretching and range of mobility.
Skeptic: And what can we do to keep our minds healthy?
Price: I think that we have really been on the wrong track in Alzheimer’s, for a long time.
First, people almost always say amyloid plaques cause Alzheimer’s. I don’t think that’s true. I think the biggest factor is metabolism and what the brain has to do to maintain energy. Your brain is only two percent of your body’s biomass, but it uses 20 percent of your body’s energy. That’s 10 times more metabolically demanding than the body average. So, every second of every day, you’ve got to supply it with energy. As you get older, your ability to perfuse oxygen into your brain through your blood vessels goes down. It decreases, just like muscle mass.
As that happens, certain regions of the brain become lower in oxygen. The amount of energy that you can create goes below the amount that you need, so certain neurons start dying. As they die, you put more demand on the remaining neurons. Their demand goes up while their supply stays the same. Then they die, which puts even more pressure on those remaining. So you get this cascade of cell death.
The second big factor that everyone knows about is that if you have the gene APOE, you have a high risk of Alzheimer’s. How can your neurons keep making a lot of energy under these low-oxygen conditions? Supporting cells, called astrocytes, are really important here. There is a 9:1 ratio of astrocytes to neurons, and they support energy generation in the neurons. So you want to keep the cholesterol level in the astrocytes low in order to keep energy generation high in neurons under low oxygen conditions.
APOE has a role in the transport of cholesterol out of astrocytes. APOE4 does it slowly, APOE2 does it fast. And if all you do is take those two facts—the lowering of oxygen and the difference in the efficiency of keeping positive energy balance under that condition—you can recapitulate the ages at which all the different genotypes get Alzheimer’s disease with very close accuracy. That’s true for a whole range of genetic and environmental backgrounds.

This article appeared in Skeptic magazine 28.4
Buy print edition
Buy digital edition
Subscribe to print edition
Subscribe to digital edition
Download our app
Then there’s a gene called TREM2 that has to do with the energetics of what’s required to clear debris. If these cells are dying, you get all this debris that you’ve got to clean out. That comes at an energetic cost. And if you spend energy doing that, you don’t have as much energy left to protect the nerve. As your neurons die and you lose synapses, as that synapse firing goes down, you cross a threshold where you can no longer do what’s called Hebbian learning—what fires together, wires together. But you don’t have enough firing to learn, so the brain has to secrete a molecule in order to recruit additional synapses— amyloid beta. It is brought in by the brain or made by the brain in order to recruit these synapses so you can keep cognition going. Now, as a byproduct, these things glom together and form amyloid plaques. Amyloid can embed in your blood vessels and constrict them, which then gets us back to the central problem of limited energy because it’s limiting oxygenation into the brain. But the plaques aren’t the cause of the disease.
Skeptic: So, what can be done to prevent that or treat it?
Price: Exercise. Under the model I just described, it’s obvious why.
Skeptic: How do you see the future of health and wellness?
Hood: I think any brand-new idea almost never can be achieved in the context of an existing bureaucracy. Bureaucracies are honed by the past, can barely deal with the present, and have difficulty dealing with the future. Initially, 80 percent of the biologists in the U.S. were opposed to the Human Genome Project.
As a young assistant professor at Caltech, I started thinking about where I wanted my future scientific career. I had a real interest in human biology and disease. It was 1970 and I was dismayed by the complexity of the problem and by the lack of tools we had for dealing with it. I decided to develop instruments that allowed one to read and write DNA, which could decipher that complexity by generating big data from individual humans. Put simply, lots of information on individuals that—when analyzed—could lead to insights into wellness and prevention.
I hope we were able to convince you that this sort of thinking is absolutely mandatory for improving healthcare in the future, and that scientific research is now on the right track. ![]()
This print interview has been edited from a longer conversation on The Michael Shermer Show.
About the Interviewees
Leroy Hood developed the DNA sequencer which enabled the reading of the entire human genetic code as part of the Human Genome Project. His work was also instrumental in the creation of treatment for AIDS. Hood founded the discipline of systems biology and is one of only 15 individuals elected to all three U.S. National Academies (the National Academy of Science, the National Academy of Engineering, and the Institute of Medicine).
Nathan Price is Chief Science Officer of Thorne HealthTech and Professor at the Institute for Systems Biology. Selected as an Emerging Leader in Health and Medicine by the National Academy of Medicine, he received the Grace A. Goldsmith Award for his work on scientific wellness, and is the author of more than 200 peer-reviewed scientific publications
This article was published on March 8, 2024.